Does Sif4 Have a Dipole Moment
Correspondingly what kind of bond is SiF4. First of all a dipole moment is when the overall molecule is polar and interacts with other molecules or compounds with an intermolecular force of dipole-dipole or dipole-ion.

Which One Of The Following Pairs Of Molecules Will Have Permanent Dipole Moments For Both Members Target Batch
SiF4 is tetrahedral so that the individual dipoles on the Si-F bonds cancel and the molecule has no dipole moment.

. BeCl2 BF3 AsCl5 CO2 SiF4. The answer is SiF4. Does SiF4 have dipole-dipole.
Structure of SiF4 is a regular tetrahedron any regular geometry has net zero dipole moment as all individual dipole in a molecule cancel the effect of. Which compound below contains polar bonds and has a permanent dipole moment. SO3 is trigonal planar so that the individual dipoles on the S-O bonds cancel and the molecule has no dipole moment.
Because the Oxygen atoms in this one becomes more elctronegative and the other becomes electropositive and hence the dipole. Its going to form a pyramid structure with the silicon in the middle the three hydrogens in a triangle in one plane and the fluorine at the apex opposite the three hydrogens. SF6 OF2 ClF PF3 SiF4 SiF4.
Chemistry questions and answers. Image 4 Hence the correct. Dipole moment is the measurement of polarity of a bond.
Thus the SiF4 molecule has zero dipole charge. SF4 has a larger dipole moment than SiF4. SiF4 is tetrahedral so that the individual dipoles on the Si-F bonds cancel and the molecule has no dipole moment.
Hence it does not have a permanent dipole moment. -has a planar structure so it is non-polar. If the molecule has some net dipole moment then it means the molecule is over all polar.
The tetrahedral geometry is symmetrical and hence polarities of the Si-F bond cancel each other. What is an example of dipole-dipole. The octet rule is violated by at least one atom in all the following compounds exceptSF6 PF6- BrF5 ICl2- SiF4 CF3-The lewis formula of which species does.
Answer 1 of 2. SiF4 has the same molecular geometrical structure as carbon tetrachloride CCl4 only different is here silicon in place of carbon and fluorine in the place of chlorine. Yes both of them have permanent dipole moment.
It leads to zero net dipole moment of the silicon tetrafluoride. Answer 1 of 2. The dipole moment is defined as the products of induced charge and distance of separation between the atoms.
The dipole moments cancel each other.
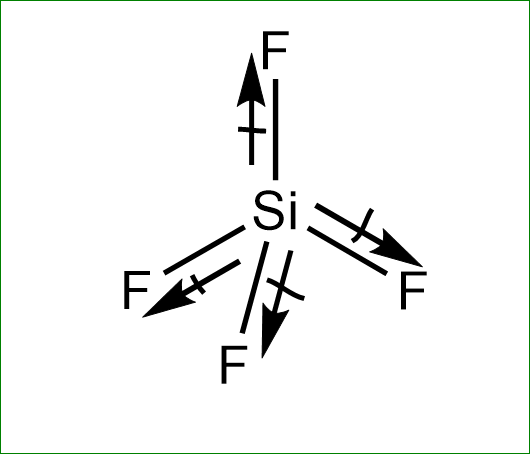
Molecule Having A Non Zero Dipole Moment A Sf4 B Sif4 Class 11 Chemistry Cbse
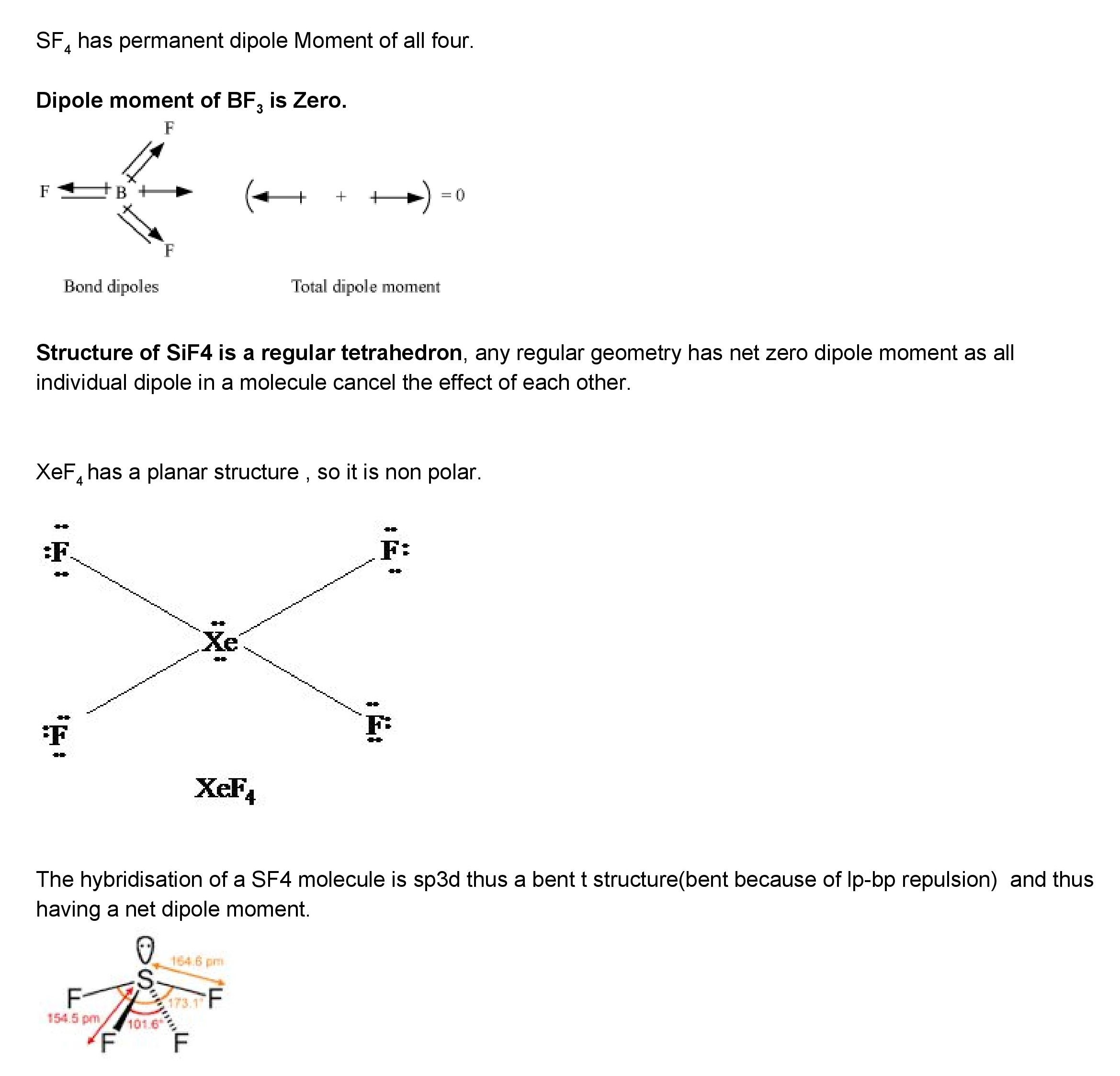
Which Of The Following Would Have A Permanent Dipole Moment 1 Bf3 2 Sf4 3 Sif4 4 Xef4

Comments
Post a Comment